The current position: Home > Latest News > Asymmetric catalysis mounts Science >

The development of robust stereoselective synthesis methods that enable precise control over the simultaneous construction of multiple stereocenters is critical for accelerating the discovery of next-generation drugs. The increased stereochemical complexity of these compounds has inspired academic and industrial chemists to invent modern and more efficient methods to facilitate their construction. Enantioselective catalysis is a powerful and broadly applicable paradigm that has been widely used, and the availability of countless mechanical manifolds for the construction of C-C and C-heteroatom bonds makes this blueprint particularly attractive. With notable exceptions, discoveries in enantioselective catalytic reactions tend to focus on how to maximize stereoselectivity (transition state focus), while issues surrounding post-reaction workup and optimization of product physical properties (ground state focus), in areas such as polymers Critical chemistry, or process chemistry, is often overlooked. An unfortunate by-product of this focus on bifurcation is that even for relatively efficient reactions, practicing chemists often face laborious energy and resource-intensive purifications, which limit applicability on a larger scale. This problem is exacerbated when valuable substances are lost as unwanted stereoisomers.
The discovery and development of catalyst-mediated asymmetric Michael addition/crystallization-induced wide-ranging diastereomeric transformations is reported by Jeffrey S. Johnson, Department of Chemistry, University of North Carolina at Chapel Hill. This sequence controls three stereocenters, two of which are stereochemically unstable. Configurational instability of 1,3-dicarbonyl and nitroalkanes, often considered a disadvantage in stereoselective syntheses, enantioselective Bronsted base organocatalysis and thermodynamic stereotyping by using single convergent crystallization Combined with controls, this can be used effectively. The related results were published in Science as "Doubly stereoconvergent crystallization enabled by asymmetric catalysis".
。

图 1。提出的机制和初步研究。
图 2。结晶化立体收敛共轭加成的底物范围。
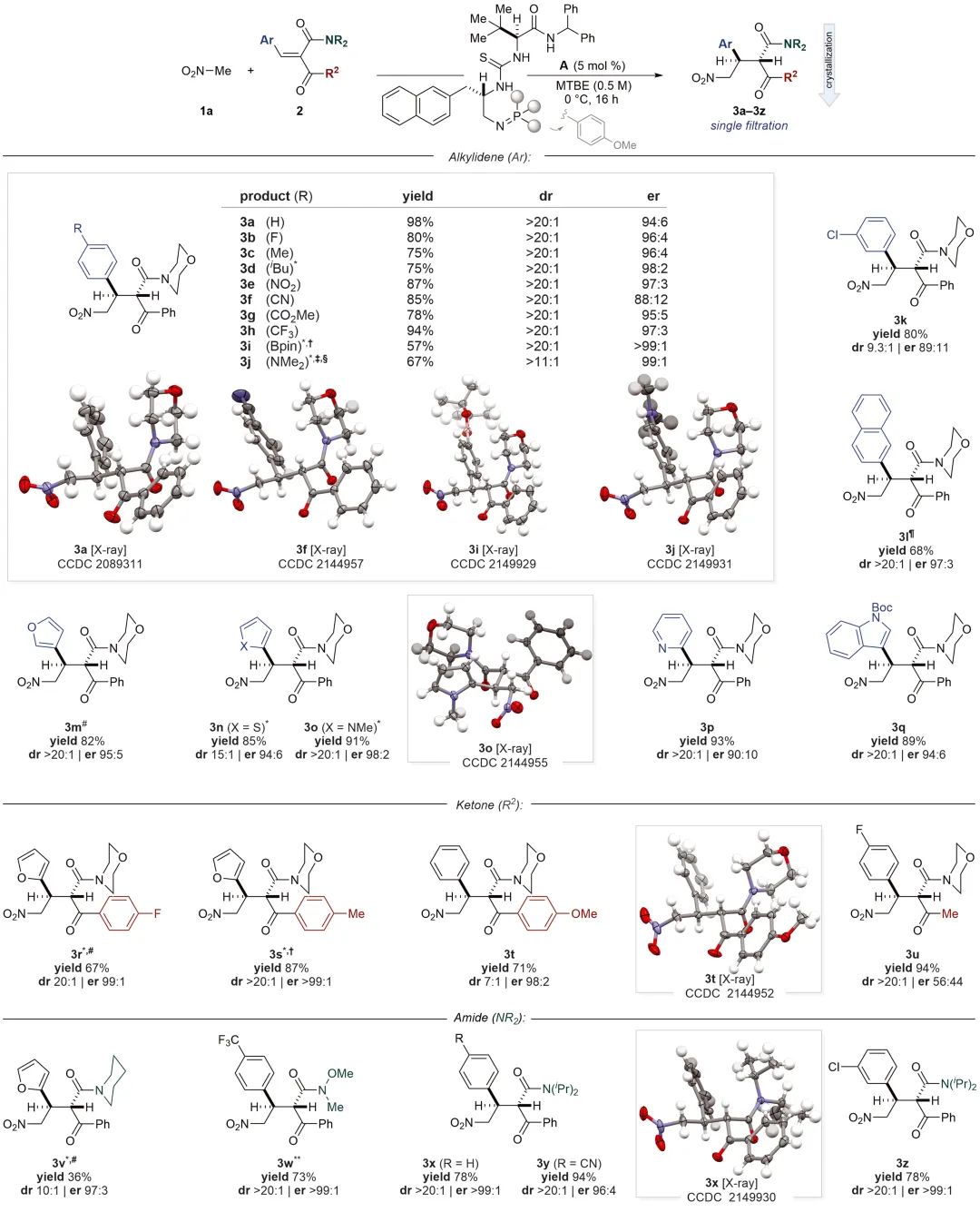
图 3。结晶双立体会聚共轭加成的底物范围。

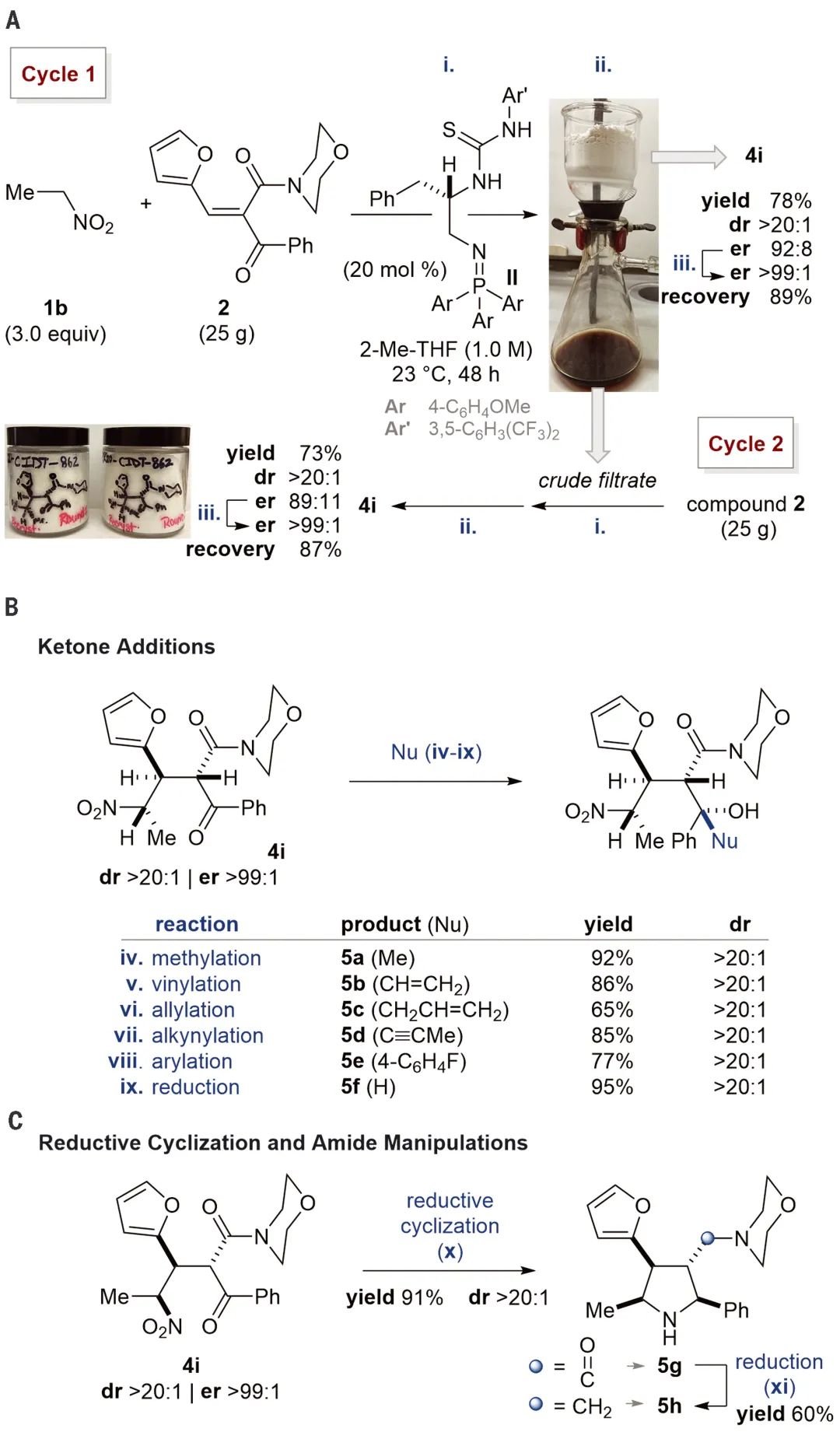
图 4。对立体选择性起源的机理见解。
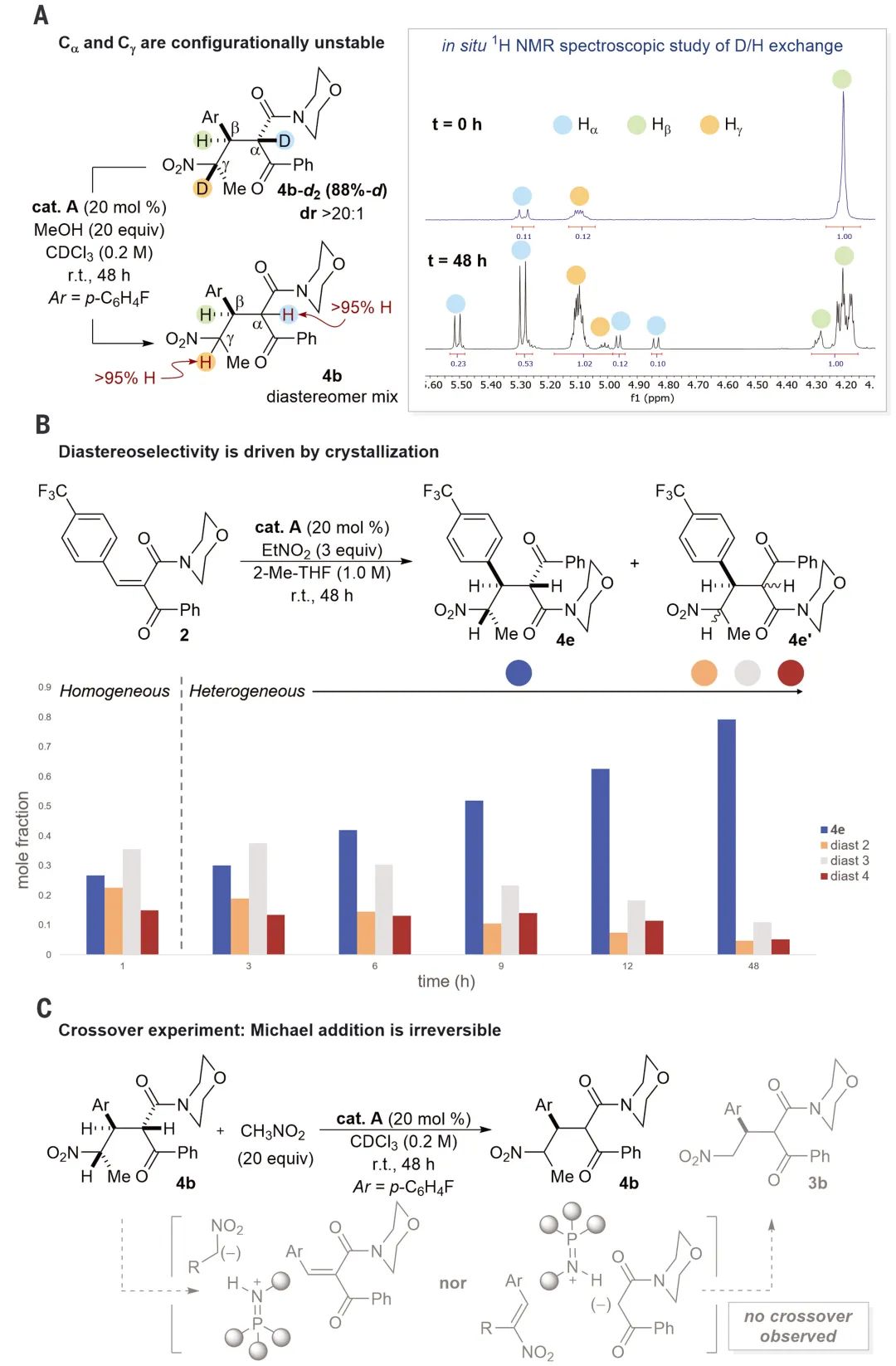
图 5。结晶双立体会聚共轭加成的合成效用。